All articles by Adam Zamecnik

Adam Zamecnik
@a_zamecnik
Adam Zamecnik is a healthcare reporter for Pharmaceutical Technology. He has covered diverse topics such as continuous drug manufacturing, genomic research and sexual health. He is particularly interested in new technologies, health inequality, and cell and gene therapy research.
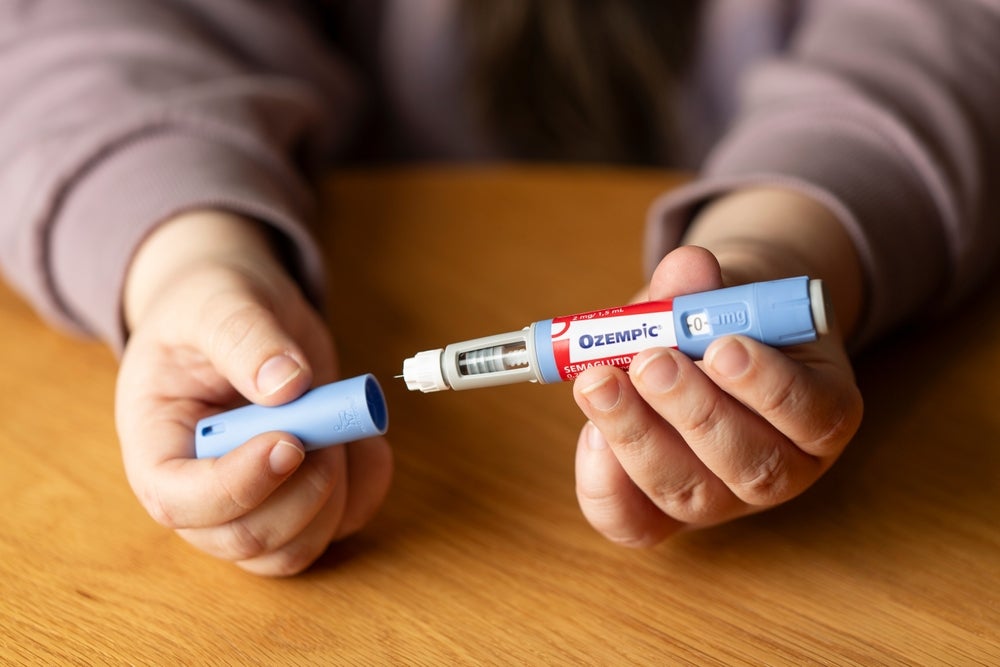
Q&A: Tackling the rise of fake anti-obesity drugs on the market
Professor Timothy Mackey talks about the recent reports of fraudulent Ozempic pens and the challenges in chasing drug counterfeiters.
CTO Europe 2023: A snapshot of challenges and opportunities in oncology
Discussions at the 10th CTO Europe conference reflected old and new challenges that add pressure on companies running oncology trials.
CTO Europe 2023: EU’s IVD guidelines continue affecting early clinical work
Since its implementation in 2022, new EU regulation for IVDs remains an obstacle for companies in the precision medicine sector.
CTO Europe 2023: Fostering diverse oncology trials remains a challenge
Experts at the clinical conference continued to explore ways to improve representation in clinical trials and the regulatory push behind it.
CTO Europe 2023: Reflections on drug development through the phases
Closing the first day of the CTO Europe conference, a global CRO shares insights on the drug development lifecycle in oncology.
CTO Europe 2023: Cancer clinical trials shift as sector goes digital
Opening the first day of the CTO Europe conference, experts set their sights on the impact of digital technologies on oncology trials.
How can smaller biotechs navigate the landscape of oncology trials?
An upcoming two-day event will host talks on the impact of new technology on oncology trials and smaller biotechs.
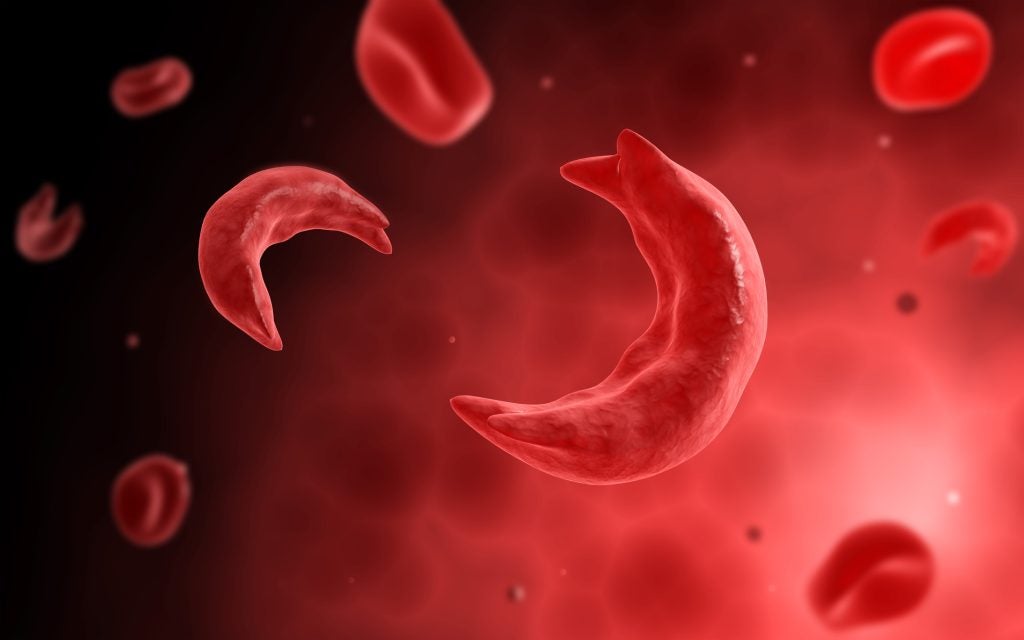
How gene therapies can transform sickle cell disease treatment
The potential for using gene therapies to treat sickle cell disease is high, but their durability and high price point invite scrutiny.
CymaBay maps out regulatory filing for primary biliary cholangitis drug seladelpar
Regulatory applications in EU and the UK will follow a potential filing in the US in early 2024.
Kinnate Biopharma announces 70% layoffs and pipeline restructuring
The company will suspend the development of three assets, considering strategic alternatives for two clinical programmes.