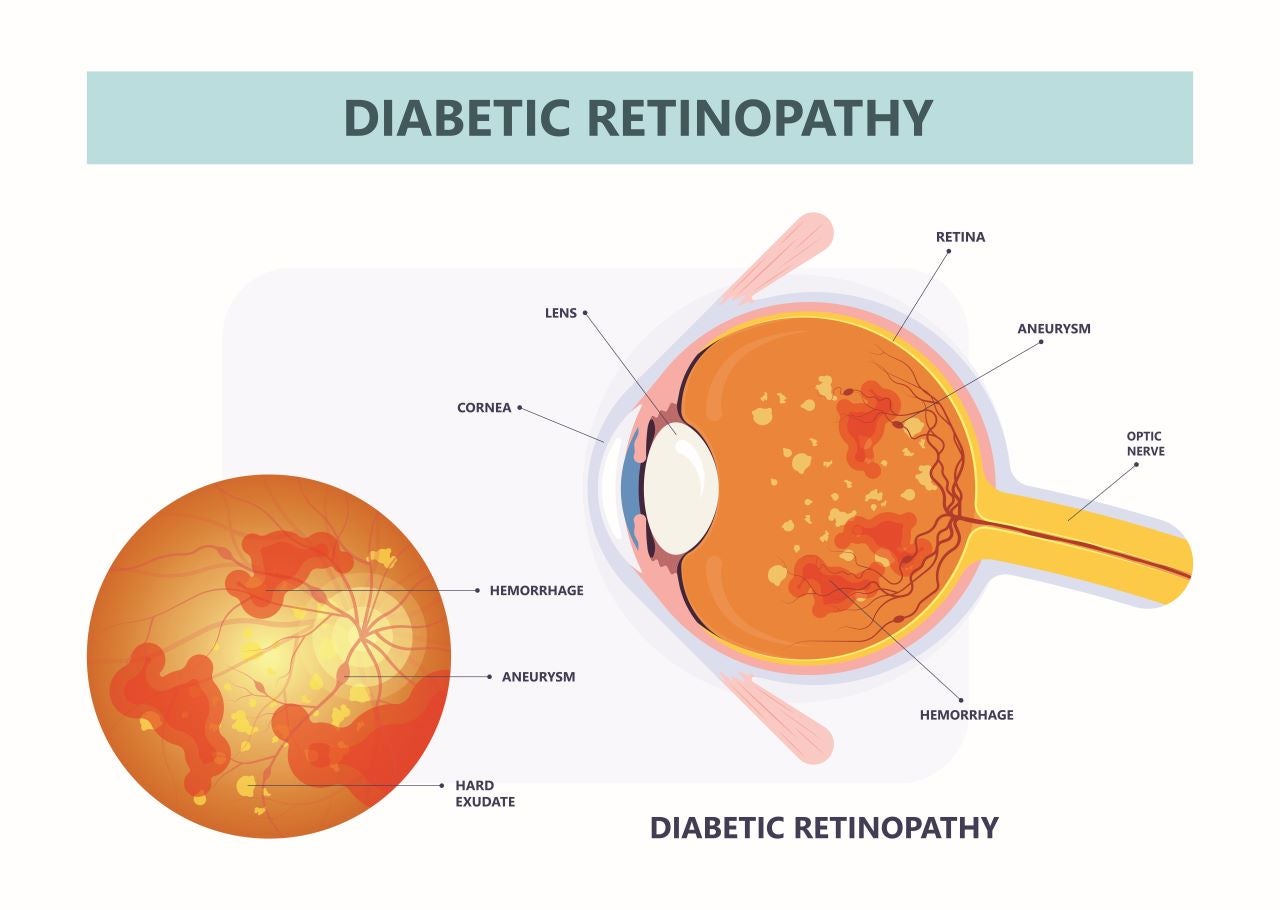
At the 127th Annual Meeting of the American Academy of Ophthalmology (AAO) 2023, results were presented from the Phase II ALTITUDE trial, evaluating AbbVie and RegenxBio’s ABBV-RGX-314 for the treatment of diabetic retinopathy (DR) at dose levels one and two. ALTITUDE provided results on the efficacy and safety of ABBV-RGX-314 over the course of 12 months. ABBV-RGX is one of the first highly anticipated gene therapies within the retinal disease space.
In addition to its application within DR, it is also in Phase III for age-related macular degeneration (AMD) and in Phase II for diabetic macular edema (DME). In the next five years, the DR patient population is expected to reach 20 million across the US, Europe and Japan. Less than 1% of patients are currently getting treatment for early DR due to the high treatment burden associated with the disease. Although there are many approved therapies for the treatment of DR, many of these treatments are invasive and require repeated interventions. The risk of patients with DR progressing to DME within five years is 45% for moderate non-proliferative diabetic retinopathy (NPDR) and 62% for severe NPDR. Therefore, the development of therapy with more durability and a lesser treatment burden is of utmost importance to prevent the progression of DR.
ABBV-RGX-314 comprises a gene therapy vector encoding the anti-vascular endothelial growth factor (VEGF) fab gene via AAV8, as this vector best transfects the retinal pigment epithelium to facilitate long-term anti-VEGF expression in the eye. The purpose of this gene therapy is to stabilise and provide a long-term improvement in the severity of DR and decrease the chance of vision loss, namely loss in best-corrected visual acuity (BCVA) via a one-time injection. The gene therapy requires a suprachoroidal route of administration in the clinic.
Patients were administered ABBV-RGX-314 on day one of the study at dose level one (2.5 × 10¹¹ GC/eye), which was administered to COHORT 1 (comprising 15 subjects who were administered ABBV-RGX-314 NAb and five subjects who were observational controls), or dose level two (5.0 × 10¹¹; GC/eye), which was administered to COHORT 2 (comprising 15 subjects who were administered ABBV-RGX-314 NAb and five subjects who were observational controls) and COHORT 3 (comprising 20 subjects who were administered ABBV-RGX-314 NAb+). No prophylactic steroids were administered within these three groups.
Arshad Khanani, MD, Clinical Associate Professor at the University of Nevada and Managing Partner, Director of Clinical Research and Director of Fellowship at Sierra Eye Associates, reported the results of this trial with regard to efficacy. The trial results showed that over the course of one year, in all patients at dose level two, 2.9% of patients had a one-step worsening in DRSS, 8.6% of patients presented with a two-step worsening in DRSS, 34.3% of patients reported no change in DRSS, 34.3% of patients showed a one-step improvement in DRSS, 14.3% of patients showed a two-step improvement in DRSS and 5.3% of patients showed a three-step improvement in DRSS.
As for the NPDR-only patients, no worsening of DRSS was reported at dose level two. A total of 29.2% of patients had no change in DRSS, whereas 50.5% of patients showed a one-step improvement in DRSS and 20.8% of patients showed a two-step improvement in DRSS. In the dose level one group, 66.7% of patients had an improvement in DRSS, whereas in the dose level two group, 70.8% of patients had an improvement in DRSS.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalDataFurthermore, in terms of vision-threatening events (VTEs), 37.8% of patients in the control group had VTEs, 16.7% of patients had VTEs in the dose level one group and 4.2% of patients had VTEs in the dose level two group. Therefore, a reduction of 89% in VTEs was seen in the patients treated with dose level two in comparison to the control group, showing that the disease is being stabilised and treated in patients who received ABBV-RGX-314.
ABBV-RGX-314’s safety profile was the primary goal of the study. Results of ALTITUDE showed that, with regards to treatment emergent adverse events across both dose levels, 30% of patients had conjunctival hyperemia, 14% had a conjunctival haemorrhage, 12% had mild to moderate episcleritis that was resolved with topical corticosteroids, 8% had an increase in intraocular pressure (IOP) and 6% had mild intraocular inflammation that was resolved with topical corticosteroids. Seven serious adverse events were reported, but none of them were related to ABBV-RGX-314. Nonetheless, no cases of occlusion, chorioretinitis, vasculitis, or hypotony were reported and BCVA was stable at one year. Therefore, results showed that at both dose levels, ABBV-RGX-314 was well tolerated by patients.
Thus, this study proved that with a single injection of ABBV-RGX-314, improvements in BCVA were maintained for up to one year. Moreover, disease progression was prevented in all patients with NPDR within the dose level two group and VTEs were reduced by 89% in NPDR patients compared to those in the control group. The study also confirmed that ABBV-RGX-314 was safe and well tolerated by patients at both dose levels.
Key opinion leaders (KOLs) interviewed by GlobalData have expressed great interest in gene therapy and believe that it has the potential to be a game changer for retinal diseases and may eliminate the need for injections. Gene therapy is largely the future of treatments for retinal indications, including AMD and vein occlusions. Meanwhile, some KOLs are slightly apprehensive, as they believe that chronic suppression of VEGF is not a good approach for diabetic patients. Nonetheless, gene therapy is being awaited with a general sense of great excitement for many retinal diseases, especially DR.
According to GlobalData’s Pharma Intelligence Center, there are 40 Phase III candidates, 49 Phase II candidates and 46 Phase I candidates for AMD globally. There are 13 Phase III candidates, 30 Phase II candidates and 17 Phase I candidates for DME globally.







Related Company Profiles
AbbVie Inc
RegenxBio Inc