The Bristol Myers Squibb East Syracuse campus is more than 60 years old. Launched in 1943, the pharmaceutical manufacturing facility made nearly 70% of the penicillin produced in the world until the mid-2000s. Initiated in 2010, the project aimed to transform the site from a penicillin-manufacturing facility into a park-type biotech research centre. It was completed in 2013, reflecting the company’s overall strategy.
The project took nearly three years to complete. It involved the demolition of 50 buildings on the campus, which have become obsolete and remained idle since penicillin production was phased out in mid-2000s. The project was completed at a cost of more than $10m.
East Syracuse facility
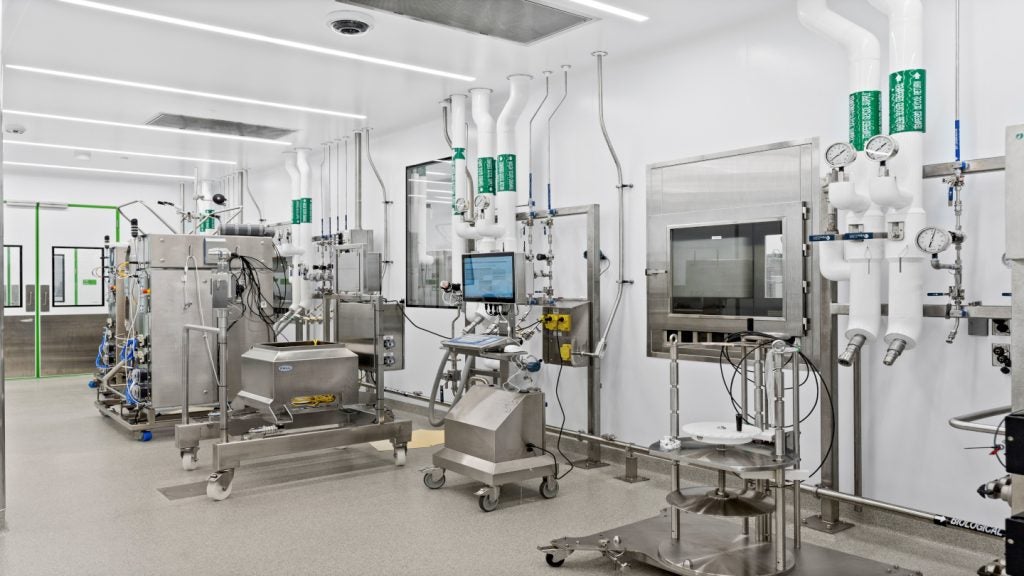
The site is a large campus of 118 buildings. Spread over 90 acres, it accommodates vital facilities such as the latest biotechnology process development laboratories. There is about 1.2 million square feet of space dedicated to administration, research and manufacturing. State-of-the-art equipment within the facility includes 5,000l fermentation bioreactors.
Bristol Myers project
The first phase in the site’s transformation established green areas and demolish structures related to previous antibiotic manufacturing. Buildings that accommodate laboratories, administration and manufacturing, occupying more than 500,000ft² of space were torn down during this phase.
The demolition freed nearly 42% of the total square footage of the campus. The existing laboratories were renovated to host wet chemistry and instrument labs. Four buildings, including chemical laboratories and office areas, were also combined.
The proposed second phase is expected to oversee the end of more obsolete structures. If approved, it will include the demolition of the administration building at the plant’s entrance from Thompson Road, which is one of the few buildings visible to the public from the road. A total of 150,000ft² of space will be demolished.
The project reduces the facility’s total operating expenditure and total energy consumption. Other green elements of the project include reusing and recycling the materials. However, no new structures were built as part of the project.
Contractors
The lead contractor for the project is Hueber-Breuer Construction Co. The contract included design and construction services. The firm undertook activities such as expanding good manufacturing compliance, and adding capacities for simultaneous production and bioreactor harvest with centrifuge technology. It also renovated the utilities and support systems within the facility.
Antibiotic production
For decades, the facility has developed a bulk active antibiotic that constitutes the active pharmaceutical ingredient for producing penicillin. However, in the last decade, the facility’s operations have shifted to developing processes to manufacture biologics, or medicines made from plant and animal-derived cells.
The facility has the capacity to make biologic medicines for clinical trails and commercial drug launch. Full-scale production can be carried out in smaller volumes in the Syracuse facility. Large-scale production will be performed at other Bristol Myers facilities.
Cell technology
The Syracuse facility develops biologics by growing animal or plant-derived cells in highly controlled environments. The cells are mixed with the correct amount of oxygen and nutrients and kept for the right time limit, temperature and pH.
During the process, the cells produce proteins that are harvested, purified and made into biologic drugs that treat a host of diseases, including cancer, arthritis and diabetes.