Cambridge Major Laboratories (CML) opened a new active pharmaceutical ingredient (API) facility in July 2009. Located in Germantown, Wisconsin, USA, the facility has been constructed in response to CML’s growing capacity requirements to support its increasing pipeline of developmental and commercial manufacturing projects.
The facility offers a US-based high-quality API supply option to clients in the pharmaceutical and biotechnology industries. The facility began construction in May 2008 and was completed in just over a year.
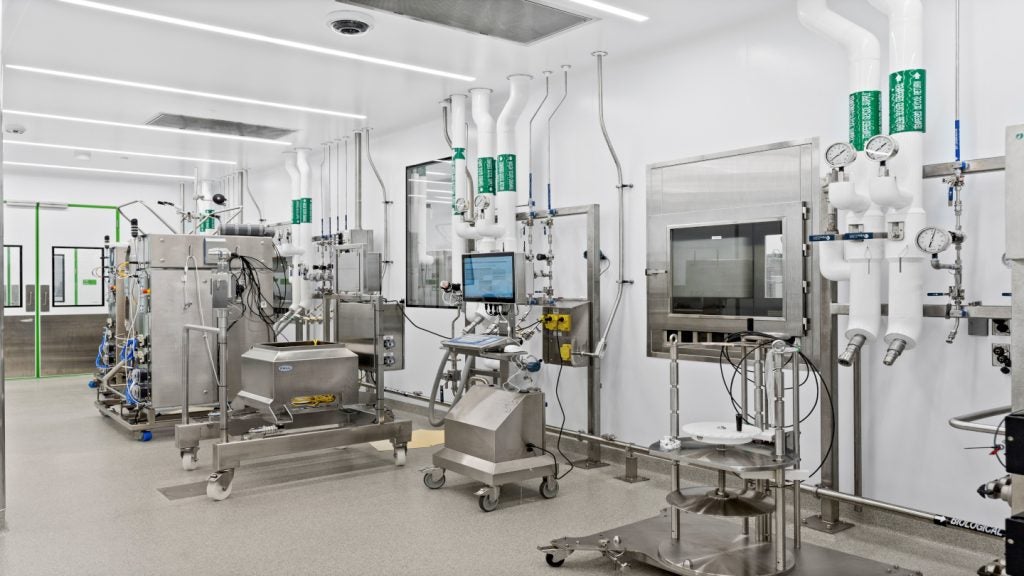
CML’s Germantown facility
The facility is a 125,000ft² multi-storey building housing six good manufacturing practice (GMP) suites. Designed to produce both API and advanced pharmaceutical intermediates in multi-ton volumes, the suites operate at a total installed capacity of 18,000 gal. Each suite is equipped with a different combination of vessel sizes and / or metallurgies. The suites address changing process requirements.
Two suites, designated as third and fourth, are equipped with two 2,000 gal and one 1,000 gal DeDietrich Optimix GLS reactors as well as an 800mm horizontal peeler centrifuge that ensures complete product isolation and drying. Both suites are additionally equipped with Kraus-Maffei helix conical dryers with 800l and 900l capacity.
Two suites within the facility have been dedicated for hydrogenation and cryogenic reactions. The cryogenic reactions suite is installed with a 500 gal, 316L stainless-steel (316L SS) reaction vessel and a 1,000 gal 316L SS work-up vessel.
The hydrogenation suite houses a single 300 gal 316L SS hydrogenation vessel and an enclosed catalyst removal filter. Busch dry-seal pumps and liquid-ring pumps provide the vessels with high vacuum and utility vacuum capabilities.
The fifth and sixth suites are outfitted with one 1,000 gal reactor, one 1,000 gal 316 l SS reactor and plate filters. One 500 gal 316 l SS reactor and tray dryers are additionally installed within the fifth suite. The sixth suite also includes a 500 gal DeDietrich Optimix GLS reactor.
All suites are designed with total isolation and containment. To accommodate any future expansion, the suites are designed to include additional capacity of up to 30,000 gals. Thermal oxidiser technology ensures EPA and DNR air quality standards. The local scrubbers vent out all process gases before they enter the thermal oxidiser, destroying all dangerous and flammable vapours completely.
A solvent tank farm is also accomodated on-site. Equipment within the tank farm includes five 800 gal bulk solvent tanks and two 6,000 gal storage tanks for organic waste.
It is also installed with two 6,000 gal aqueous waste storage tanks.
Production and CML development at Germantown
The new facility produces API and advanced pharmaceutical intermediates. From concept studies to full commercialisation of new molecules, the new facility undertakes all stages of drug development. It accommodates projects with capacities ranging from a few grams to several tons.
CML Process technology
The Cambridge facility incorporates state-of-the-art technology for heating, ventilation and air-conditioning (HVAC) and process control. HVAC systems are monitored via a building automation system (BAS) that ensures the pressure, humidity and temperature within the processing zones are kept under control.
The BAS allows the system operator to check on the status of equipment and systems, adjust the control target points and get an alarm notification instantly. A graphic interface is provided by the administrator work station into the HVAC system. To prevent cross-contamination, each processing zone is equipped with separate air handling units with once-through technology and a significant amount of filtration.
Process control is achieved through a mix of advanced Therminol loop system and a fully automatic programmable logic controller-based control system (PLC) located in the control room. The Therminol loop system controls the temperature of the reactors, including stainless steel and glass lined.
All the reactors are capable of temperature control between -20°C and 160°C. The cryogenic system has been designed to control temperatures to -100°C.
The PLC-based control system, equipped with a graphical user interface, monitors the process and safety limits. It additionally acts as the primary medium to acquire process data.