China-based prescription drug developer and pharmaceutical manufacturer Lotus Pharmaceuticals is constructing a new headquarters building in Beijing. The new facility will be located in the Chaoyang District, within the premises of its manufacturing facility that was removed from operation in 2010.
The new headquarters broke ground on 9 March 2010. In February 2011, the company decided to expand the facility by an additional two stories at a cost of about $9m. The expansion brings the total construction cost to approximately $48m.
The project is currently in the final external and internal furnishing phase, and is slated for completion by the end of 2011.
Project
The project is part of the company’s strategy to boost its revenues by catering to the rapidly expanding pharmaceutical market in China. The company expects to generate net revenues of $150m during the first year, once the facility is fully operational.
The company’s administration office, sales office, R&D centre and production facility are currently spread across various districts of Beijing. Upon completion, the new headquarters will consolidate all operations of the company at one location, thus enabling better coordination between the departments.
Facility
The 34,000m² facility is being built on a 6,700m² parcel of land that earlier housed the company’s 50,000ft² manufacturing facility.
The entire facility will be spread over 11 storeys. Once completed, it will accommodate a manufacturing plant of good manufacturing practice standards, a research and development centre, marketing and sales centre, administrative offices and apartments for housing employees. Approximately 10,000m² (108,000ft²) of space will be dedicated to warehouses.
The headquarters building was initially designed to be a nine-floor building spanning approximately 25,000m² (269,000ft²) of area. Two new floors were added to the design to provide about 90 to 120 apartments for employees. The two floors will constitute a gross area of 9,000m² (97,000ft²). The apartments will be allotted to managerial level employees to establish a loyal and stable workforce.
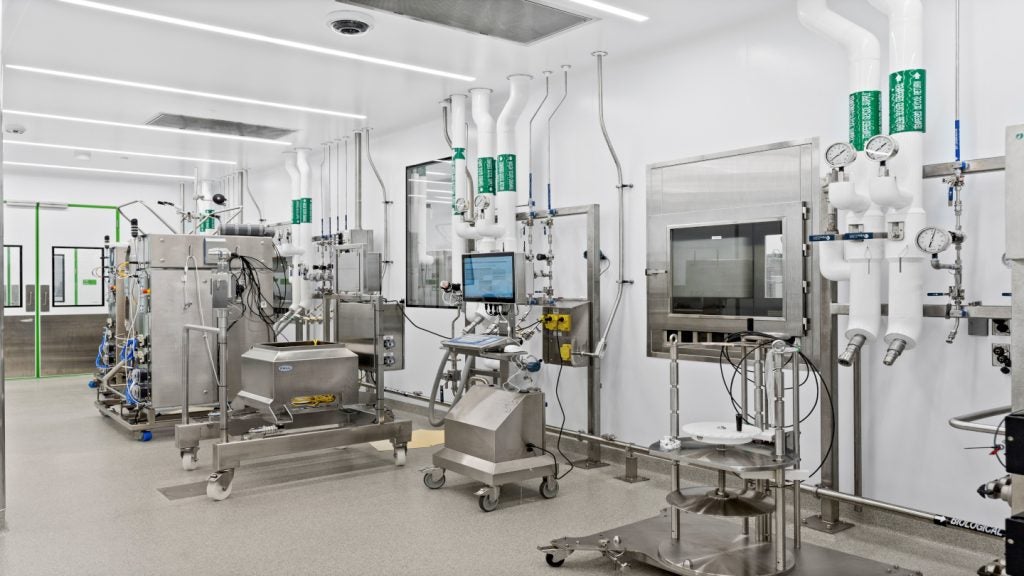
The facility will be outfitted with similar equipment to those in the previous manufacturing facility in the Chaoyang district. It will include liquid phase, gas phase, spectrum and mass spectrum equipment, and a host of purification and distilling equipment.
Construction
The Beijing Land Planning Bureau granted permission to use the land for industrial purposes after the company made full payment of the required land value increment taxes in 2009. The land was earlier permitted for green use only.
A series of events has delayed the project completion, including the temporary ban on construction projects during the National People’s Congress and Chinese People’s Political Consultative Conference in March, and multiple government inspections during several phases of construction to ensure that the facility meets the highest standards of quality.
Manufacturing
The facility will be devoted to the manufacturing, research and development of biochemical drugs. It will also develop traditional Chinese medicines, chemical compound medicines and antibiotics.
The interim drug production, until the completion of construction, has been outsourced to Shuanghe Pharmaceutical Group, who has a production facility located near to Lotus Pharmaceuticals’ existing facility.
Finance
The project is planned to be financed entirely through internal funds. Lotus Pharmaceuticals had paid for approximately $36m of the total construction cost by the end of 2010. The company currently has no plans to procure any loans for the project.
Lotus Pharmaceuticals’ Inner Mongolia Facility
The new headquarters was planned to be launched along with a new manufacturing and storage facility that was to be built in Inner Mongolia. However, the Inner Mongolia project was abandoned in order to concentrate the company’s efforts on the Beijing facility. The Inner Mongolia facility was planned to be constructed on a 167 acre site.