French pharmaceutical company Sanofi’s manufacturing plant in Goa, India, is located in the Verna Industrial Park, owned by Sanofi’s Indian arm Sanofi India, previously known as Aventis Pharma.
The facility, which performs manufacturing and packaging functions, became fully operational in 1999. An interim manufacturing facility was opened in 1997.
Sanofi’s manufacturing plant in Goa is one of Sanofi’s Pharmaceuticals Intercontinental operational units. It manufactures solid dosage forms for the Indian market, Daonil bulk tablets for the European market and Paracetamol, Cocodamol and Co-dydramol for the UK market.
The formulation development centre has 35 bioequivalence studies, in addition to 17 patents. The plant has approximately 230 employees.
Sanofi also owns a Global Development Centre in the campus. The centre develops solid dosage formulations for Sanofi’s Asia-Pacific operations.
It has another pharmaceutical and chemical plant located in Ankleshwar, Gujarat. The firm also manufactures its products at 17 toll sites.
Sanofi’s subsidiary Shantha Biotechnics operates a vaccine research and development and manufacturing facility in Hyderabad in the state of Telangana. In early 2020, Sanofi formed partnerships with the US Biomedical Advanced Research and Development Authority (BARDA), Translate Bio and GSK for the development of vaccines to treat the novel Covid-19.
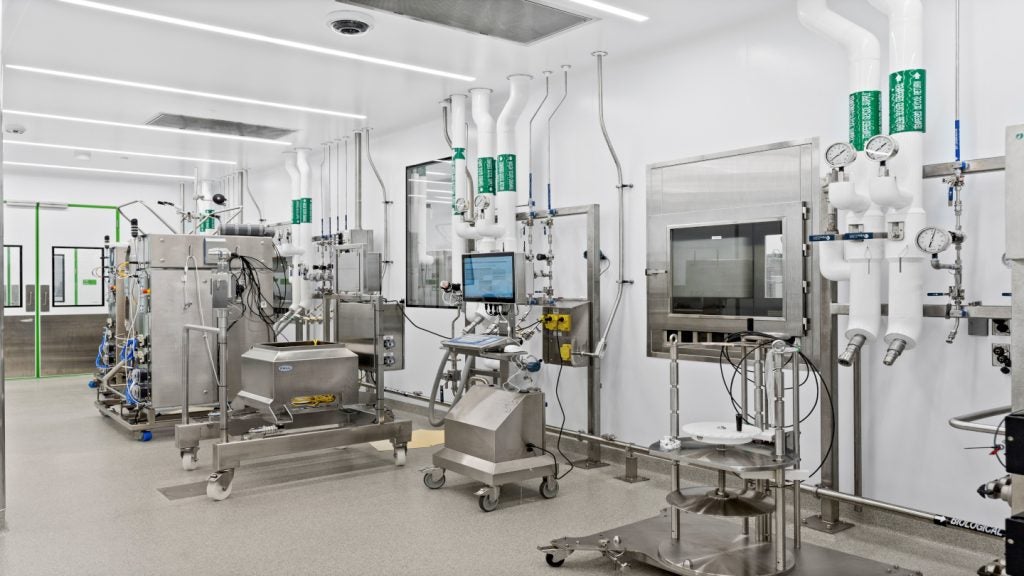
Sanofi’s Goa plant design
The Goa plant complies with good manufacturing practices (GMP) standards. It has a modular design with its components built elsewhere and brought to the site for installation. The plant has room for future expansions. Production modules are linked to a two-floor corridor.
Adhering to the health and safety environment (HSE) norms, the facility has systems in place to safeguard its personnel, property and environment. The air-handling systems were designed to ensure there is no cross contamination of products and also to protect the workers and the environment from contamination.
Separate paths were also built for the movement of personnel and material.
The facility has approvals from the World Health Organisation, Pharmaceutical Inspection Convention certified by the German Regulatory Authority, Medicines and Healthcare products Regulatory Agency of the UK, Therapeutic Goods Administration of Australia and Medicine Control Council of South Africa.
Sanofi’s Indian manufacturing plant details
The plant features fluid-bed processing equipment used for granulation of powders. It enables multistep granulation, which involves pre-heating, granulating and drying of the powders. A quality control lab along with packing material and artwork design machinery are included in the facility.
Sanofi-aventis global development centre
Sanofi-aventis, the vaccines division of Sanofi, opened its global development centre (GDC) on the Goa premises in December 2007. It is the company’s first pharmaceutical development centre in Asia. It is also the group’s single largest investment in India at €15m.
The 2,600m² centre supports the group in introducing new medicines in Asia-Pacific. The centre is used for analytical and pharmaceutical formulations and has a capacity to develop 12 compounds a year.
Sanofi opened the centre with a view to expand its presence in the region and tap the local markets. It also provides impetus to worldwide formulation efforts of the group.
Products from Sanofi’s manufacturing plant in Goa
Sanofi’s manufacturing plant in Goa produces Amaryl and Daonil tablets for blood glucose reduction, Cardace for hypertension and congestive heart failure after acute myocardial infarction, Frisium for seizures and Stilnoct for insomnia. All these are targeted at the local market.
Bulk Daonil tablets are exported to Spain, Canada and South Africa. Pain relief medicine Panadiene is exported to Singapore, Malaysia and Hong Kong.
The plant also exports pain relief drugs Co-drydamol, Mersyndol and Paracetemol.
Marketing commentary on Sanofi
In India, Sanofi operates through five entities − Sanofi India, Sanofi-Synthelabo (India), Sanofi Pasteur India, Shantha Biotechnics and Genzyme India. It has more than 5,000 employees in the country.
Sanofi has been operating in India since 1956.
The company offers 360-degree solutions from large-scale manufacturing of pharmaceutical dosage forms and vaccines, to formulation development and API development. It produces ten billion tablets annually.
Sanofi India acquired vaccine manufacturer Shantha Biotechnics in 2009. The Goa plant executed measure to reduce energy usage in 2018 and received approval to co-process hazardous waste via a cement industry.
Aventis Pharma bought the nutraceutical formulations business of India-based Universal Medicare in August 2011.
The Universal Medicare products acquired by Aventis have annual sales of INR1bn ($20m).