CXV Global provides technology leading manufacturing and packaging solutions to high-end research and development (R&D) and manufacturing sites for assistance in pharmaceutical supply chains.
We deliver core technology-leading solutions in the areas of IT and real-time automation, machine vision, serialisation, digital transformation, and professional and managed services to global customers.
Customers trust CXV Global to work in close partnership with their multinational R&D and manufacturing teams to deploy innovative technology solutions that meet their operational and supply chain goals in a responsive and commercially measurable way.
Capabilities in the pharmaceutical sector
Our solutions comprise design, hardware, software, support and training services and provide compliance with industry regulations including the Falsified Medicines Directive, the Food and Drug Administration (FDA) Code of Federal Regulation (CFR) 21 Part 11 and Good Automated Manufacturing Practice (GAMP) standards.
CXV Global played a key role in helping our clients succeed in all aspects of the pharmaceutical industry, from drug discovery and R&D to pre-clinical and clinical operations, as well as automated manufacturing and packaging / supply chain, including regulatory and good practice (GxP) compliance.
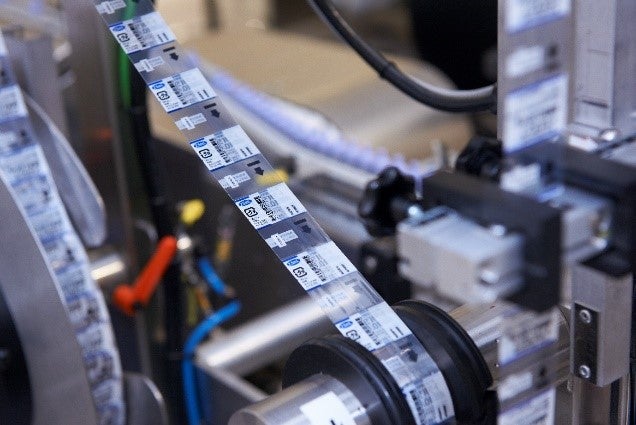
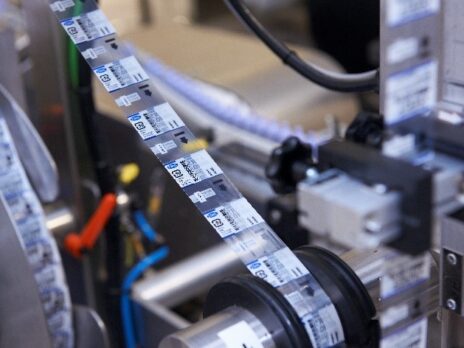
The pharmaceutical industry is secured globally through a complex network of regulations. From research and patent applications to packaging and prescribing, each stage in a drug’s lifecycle requires compliance with an associated regulation. We support compliance with constantly evolving regulations, from GAMP to anti-counterfeiting.
Expertise in serialising, resourcing and optimising packaging lines for compliance
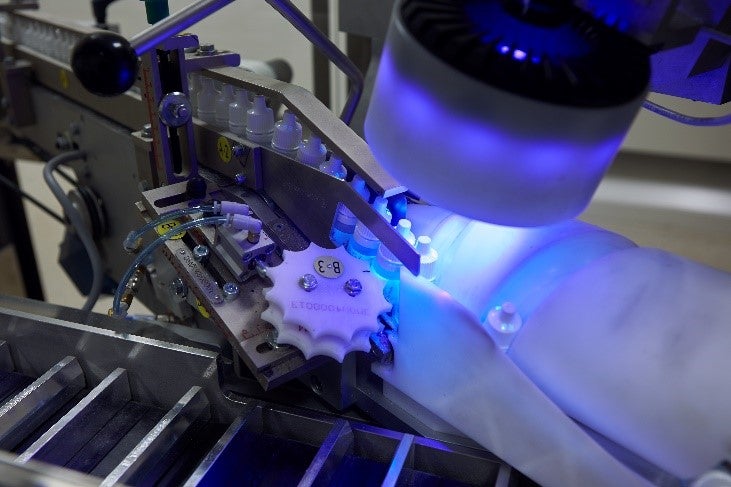
The manufacturing and packaging of pharmaceutical products are highly regulated; we serialise, resource and optimise packaging lines for compliance for companies of all sizes.
CXV Global’s relationships with our clients resulted in a broad understanding of the processes and challenges occurring in the industry. As a trusted partner, we have been assisting pharmaceutical companies in achieving compliance with regulations since 1986.
Global reach and local presence
Our combined experience and scale allow us to deliver proven technology and high-quality, local service support. Between office locations in Ireland, UK, continental Europe, North America and Latin America, CXV Global have a comprehensive network of engineers and technical management.
Our group’s engineering team are deployed across bases internationally that offer coverage across ten time zones. Our combined experience and scale allow us to deliver proven technology and effective localised support. We run projects from end-to-end, including pre-project support, design, installation, post-installation support and training.
Leading global partnerships
CXV Global partner with world-leading manufacturers of turnkey machine vision systems and machines. Our portfolio of partners includes Cognex (platinum partner), Antares Vision, Global Vision, Picomto, Universal Robots, Parsec and more.
Using our partners’ core technology, we collaborate with our customer to propose a solution optimised for their specific requirements and project delivery schedule.