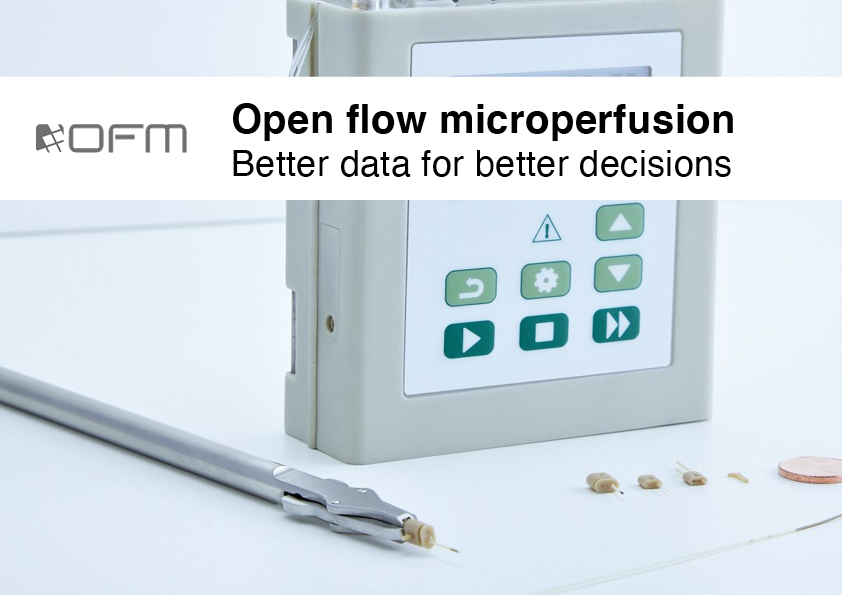
JOANNEUM RESEARCH investigates the pharmacokinetics (PK) and pharmacodynamics (PD) of novel drugs, as well as the bioequivalence (BE) of new pharmaceutical formulations at the tissue level.
From early-stage preclinical drug candidate screening to first-in-human studies and head-to-head comparisons, we support your drug development programme using our proprietary OFM technology with a focus on obtaining local PK and PD profiles directly in the tissue of interest.
Testing of dermal products
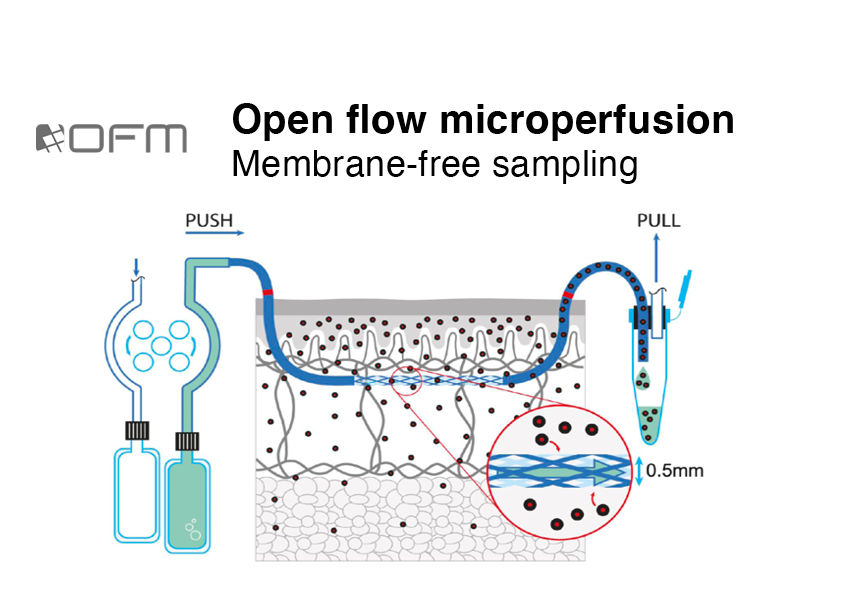
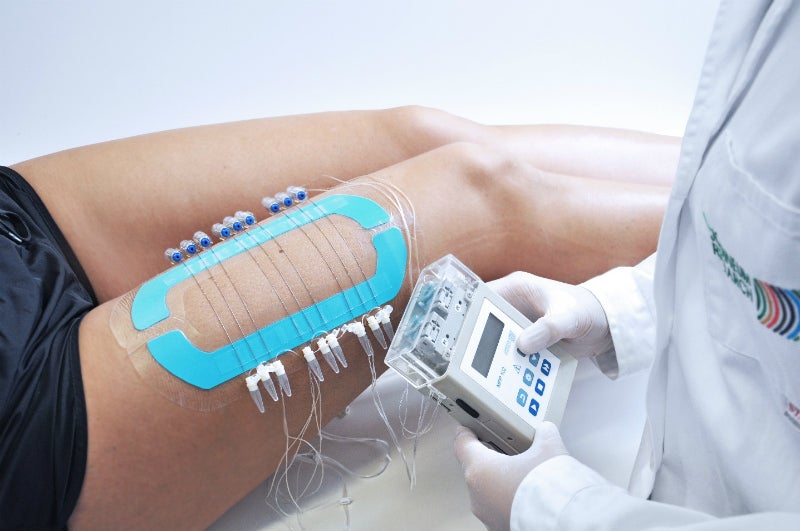
We support your dermal drug development program from early stage candidate screening to late-stage clinical studies. Using our CE-certified dermal open flow microperfusion (dOFM) technology we focus on local PK and PD profiles directly in the dermis. The same dOFM study set-up is used to compare formulations and substances ex-vivo and in-vivo thus ensuring the relevance of preclinical data for further clinical drug development.
dOFM study set-ups enable the assessment of mechanisms of action, bioavailability, penetration behaviour, release rates, inflammatory responses and immune cell reaction.
Our services include clinical studies with healthy volunteers or patients (e.g. psoriasis, chronic inflammation) as well as preclinical tests on awake or anaesthetised rodents and pigs. In our ex-vivo studies we investigate and compare skin penetration and release rates of topically applied APls (active pharmaceutical ingredient) in excised skin samples (human, porcine). Our OFM services are complemented by microdialysis services and in-vitro release tests (IVRT).
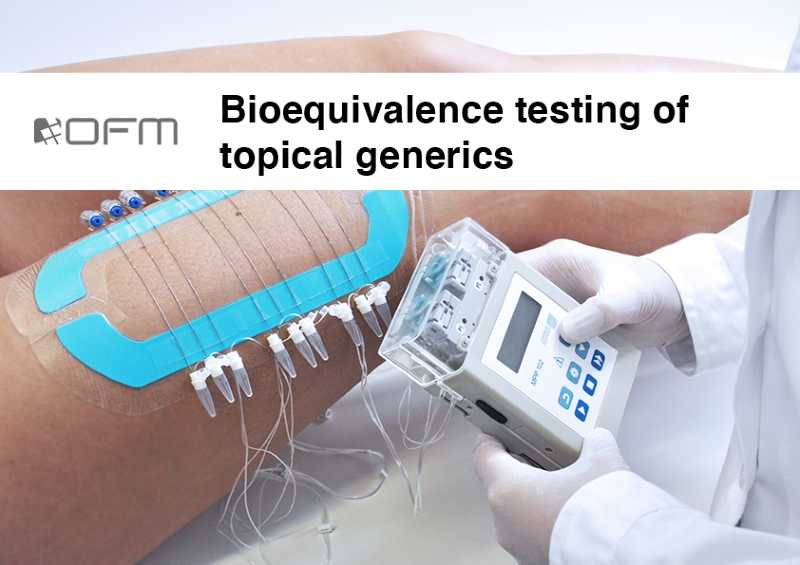
Bioequivalence testing for topical generics
Approval for topical generic drugs from the US Food and Drug Administration (FDA) currently requires a clinical endpoint study since standard blood PK studies are not useful for testing bioequivalence (BE) for topical dermal generics. dOFM allows a PK-based BE approach in the skin by monitoring substance concentrations directly in skin.
Our customers have an economic benefit by substituting expensive clinical endpoint studies with one PK study. Furthermore, our study design requires fewer participants (e.g. less than 50 healthy volunteers) in a single centre rather than a multi-centre trial involving hundreds of patients. dOFM studies can also reduce the risk of failure due to placebo effects that occur in clinical studies.
We also offer strategic consulting for product-specific guidance as well as abbreviated new drug application (ANDA) meetings.
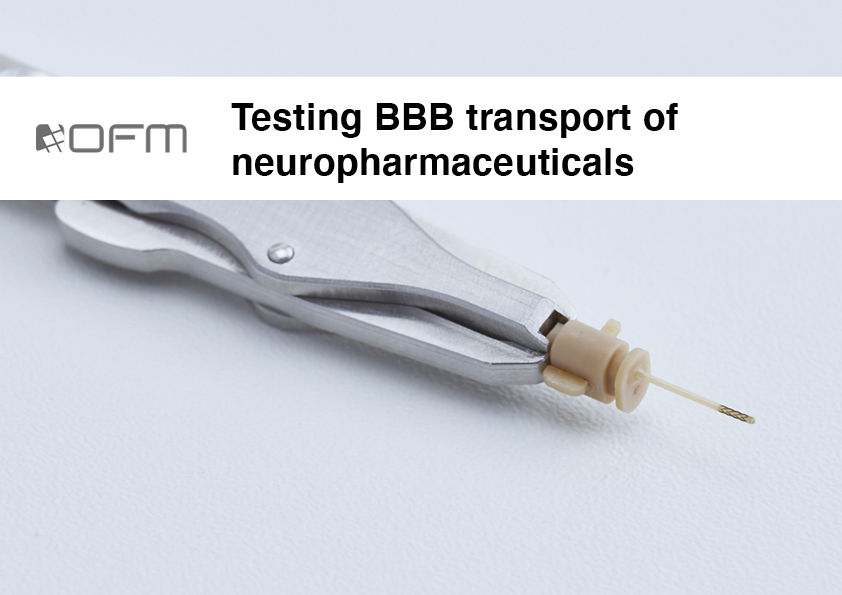
Testing in neuropharmaceutical drug development
The blood-brain barrier (BBB) protects the brain but also prevents the entry of substances for the treatment of neurological diseases. Cerebral open flow microperfusion (cOFM) allows sampling in the brain with an intact BBB for an effective feedback in drug development and offers unique insights into brain metabolism and signalling.
The most specific feature of cOFM is its capability of sampling cerebral interstitial fluid (ISF) with an intact BBB to assess the drug’s ability to cross the BBB.
Cerebral ISF samples contain all molecules present in the brain tissue, e.g. drugs, proteins and antibodies, without restriction due to size or inherent chemical properties. Furthermore, longitudinal sampling can be performed up to several weeks, which yields time-resolved, high-quality data that deliver PK and PD concentration profiles.
Benefits of cerebral OFM studies
In addition to monitoring transport across an intact BBB, cOFM can also be used to monitor neuronal biomarkers, such as leptin and tau-protein, with no limitation regarding substance size and lipophilicity, and to assess BBB function and permeability changes.
Our customers benefit from time-resolved substance concentration monitoring in the brain and sampling of highly lipophilic compounds (log P>5), antibodies and proteins. Other advantages include parallel sampling in different brain areas, application in most animal models from rodents to primates and a direct comparison of ISF with cerebrospinal fluid (CSF).
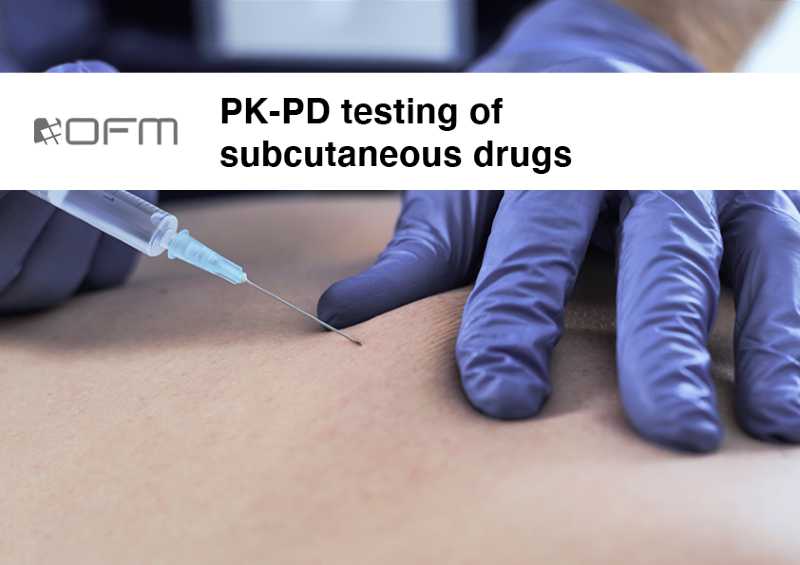
Testing of subcutaneously applied drugs
Subcutaneous injection is an application route for many substances ranging from insulin to antibodies. The formation of the liquid depot immediately after injection along with the spread of the active pharmaceutical ingredient (API) are crucial aspects that define the drug uptake into the blood.
We investigate and compare local PK and PD profiles directly in subcutaneous tissue to investigate mechanisms of action, dose-response, inflammatory markers or immune cell reaction by using standard microdialysis or our proprietary OFM technology.
Established live in-vivo imaging techniques (e.g. µCT) allow the visualisation of the injected depot in the tissue, thus providing data to optimise your injection technology and your injected formulation.