Mesa Labs offers high-quality process validation and instruments to monitor temperature, humidity, and pressure.
Mesa’s products help ensure product quality for both Fortune 500 companies and high-tech start-ups. Its systems can be used to control manufacturing processes and solve issues for pharmaceutical, medical, and food processing applications.
Mesa also offers biological indicators (BI) for medical device and pharmaceutical manufacturing sterilization monitoring, as well as DataTrace for high-quality, wireless data logging.
These products have a key technical excellence and the company holds a reputation of providing high-quality control and calibration products to ensure customer and patient safety.
Data Loggers to measure temperature, pressure, and humidity
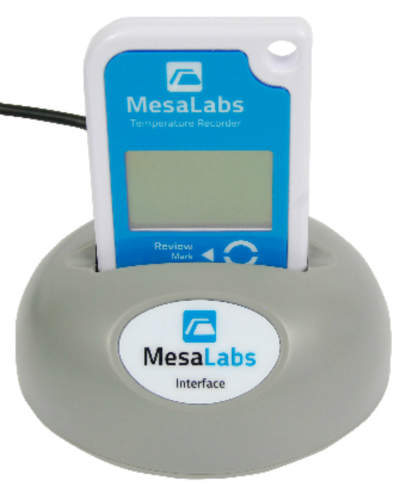
DataTrace is a high-performance, wireless data logger series manufactured by Mesa Laboratories. These devices can record temperature, humidity, and pressure, communicating this information to a server or software. A flexible system, the user can specify when a recording is logged and change the pattern at any time. Data can be accessed in a numerical or graphical form.
The device accompanies DataTrace Pro, a powerful reporting software. It can create process reports and analyze and interpret data, as well as being fully compliant with regulatory requirements. DataTrace Pro can help achieve greater insight and productivity.
DataTrace data loggers can be used to identify cold spots, validate new equipment, or establish sterility assurance.
Continuous monitoring systems
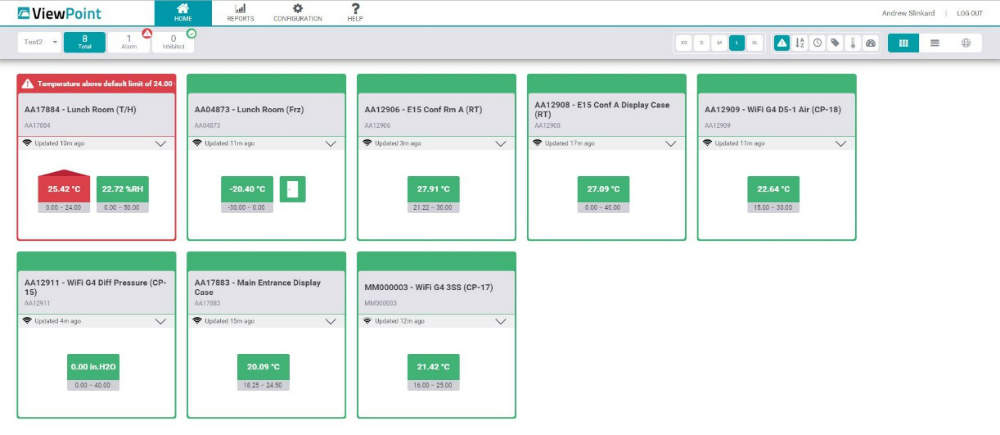
For the measurement of humidity, differential pressure, temperature, water detection, and air velocity in pharmaceutical and food processing equipment, Mesa’s ViewPoint Monitoring System can reliably provide detailed reports.
Equipment from fridges and freezers to storage containers require strict environmental thresholds, which need to be consistently monitored. ViewPoint will monitor designated equipment and send alarmed notifications, so targets are ensured and any problems are identified quickly. In this way, Mesa helps keep your facility DHS, AABB, CAP, and US Food and Drug Administration (FDA) compliant.
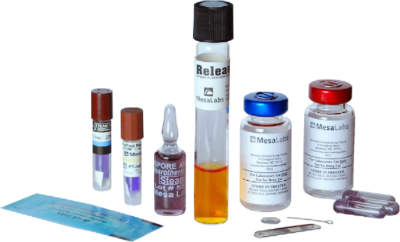
Biological indicators and sterility assurance products
Mesa’s Association for the Advancement of Medical Instrumentation (AAMI) and International Organization for Standardization (ISO) compliant BIs are designed to meet the strictest of requirements and expectations.
The company’s wide product portfolio includes Alex discs for H2O2, liquid submersible ampoules, spore suspensions, self-contained biological indicators (SCBI), specialty indicators, and spore strips. BI testing and verification of steam, ethylene oxide (EO), and other processes are also available.
Cold chain packaging
Mesa also offers TempTrust, a cold chain, validated, and qualified packaging. The complete TempTrust cold chain packaging portfolio includes qualified packaging solutions, phase change material (PCM) refrigerants, vacuum insulated panels (VIP), shippers, and portable and active solutions.
Torque analyzers
Mesa manufactures a range of durable cap torque analyzers, which produce precise results. The SureTorque and Torqo units can perform a variety of tests, as well as customized testing of a number of products. The systems are designed with various features, including injury prevention, automated controls and quick change overs.
Many caps can be tested, including child resistant caps and low-volume capping, squeezables, tamper evident, pumps, eye droppers, and desiccated caps.
Calibration and validation services
Mesa can provide a number of customizable documents to fit its clients’ validation needs. These can meet all design, regulatory, and company requirements.
On-site validation services are also available to help ensure systems, processes, and equipment is working correctly. Mesa’s testing protocol is also suited to your specific needs.
Mesa’s Calibration and Validation Services can provide:
- Installation qualification (IQ) / operational qualification (OQ) / performance qualification (PQ) services and documentation
- Warehouse mapping
- Equipment qualification and calibration
- Validation of process control
- Consulting and assessment
- Standard operating procedures (SOP) development