Respiratory
Sanofi and Regeneron’s Dupixent scores second win in Phase III COPD trial
Sanofi and Regeneron have reported positive interim data from the Phase III NOTUS trial with Dupixent (dupilumab) showing a 34%…
Janssen seeks FDA approval for lung cancer combination therapy
The Janssen Pharmaceutical Companies of Johnson & Johnson is seeking US Food and Drug Administration (FDA) approval for a Rybrevant (amivantamab-vmjw) combination…
UK MHRA extends licence for Vertex’s children’s cystic fibrosis drugs
The UK Medicines and Healthcare products Regulatory Agency (MHRA) has extended the licence of Vertex Pharmaceuticals’ cystic fibrosis drugs Kaftrio (ivacaftor,…
AstraZeneca posts 16% drop in Q3 2023 profit after tax
AstraZeneca has posted a profit after tax of $1.37bn for the third quarter (Q3) of 2023, compared with $1.64bn in…
BMS gains breakthrough therapy designation for PPF treatment
Bristol Myers Squibb (BMS) has received breakthrough therapy designation from the US Food and Drug Administration for its investigational therapy…
BioAegis scores $20m BARDA contract to develop ARDS therapy
The US Biomedical Advanced Research and Development Authority (BARDA) has awarded a $20m contract to BioAegis Therapeutics for the development…
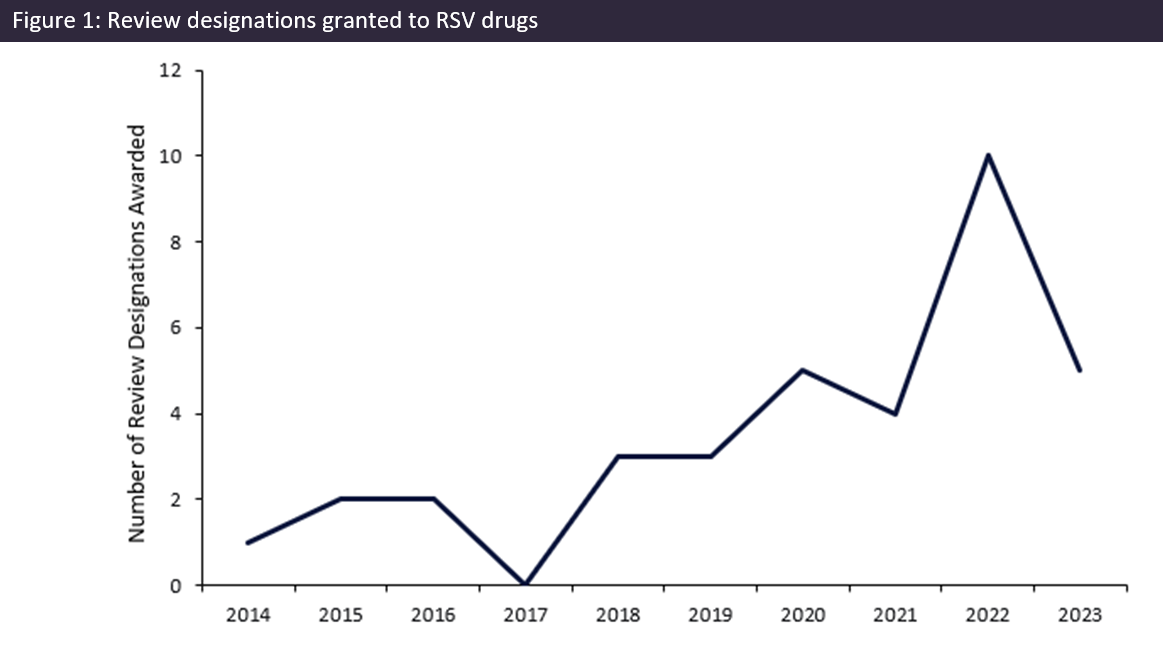
Record number of respiratory syncytial virus (RSV) designations awarded in 2022
In 2022, global regulatory authorities awarded a record ten review designations for respiratory syncytial virus (RSV)-indicated drugs. This surge acted…
Advent receives US NIH grant for bronchopulmonary dysplasia therapy
Advent Therapeutics has received a small business innovation research grant worth $3m from the US National Institutes of Health (NIH)…
MHRA approves GSK’s RSV vaccine for older adults in the UK
Older adults in the UK will soon be able to receive protection against respiratory syncytial virus (RSV) after the Medicines…
Eloxx repackages ELX-02 data in cystic fibrosis as it eyes pivotal trial
Eloxx has revealed its lead candidate ELX-02 improved predicted forced expiratory volume (ppFEV1) in patients with Class 1 cystic fibrosis…