Vertellus Biomaterials, based in the UK, is a business unit of Vertellus Specialties UK Ltd.
Vertellus Biomaterials was set up to exploit a range of clinically proven biocompatible materials known collectively as PC Technology, which was exclusively in-licensed from Biocompatibles UK Ltd in 2006.
We develop and evaluate coating methodologies to apply these biocompatible polymer treatments to a range of medical devices for worldwide collaborative partners.
PC Technology – biocompatible drug eluting coatings
PC Technology provides the performance benefits of:
- Reduced protein deposition/activation
- Reduced blood activation/thrombus formation
- Reduced bacterial adhesion
- Reduced biofilm deposition
- Reduced inflammatory response
- Reduced fibrous capsule formation
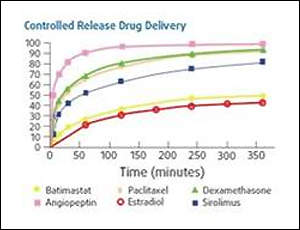
- Controlled release drug delivery
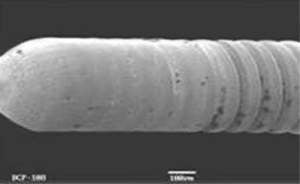
It has been used for over 15 years in the manufacture of a range of medical devices, including stents, contact lenses, catheters and cardiopulmonary bypass systems.
PC Technology materials have been shown to be excellent platforms for controlled delivery of a wide range of pharmacologically active agents. The polymers can be tailored to match the delivery requirements of a specific application. A variety of drugs have been investigated, including antibiotics, anti-inflammatories, cytotoxics, hormonally active agents, nucleic acid derivatives and therapeutic proteins.
The PC Technology is licensed to a number of leading medical device manufacturers, including CooperVision, Medtronic, Abbott Laboratories and the Sorin Group. FDA claims relating to reduced thrombus formation, reduced biofilm formation and improved patient comfort have been secured for devices incorporating PC.
PC Technology – an overview
PC is short for phosphorylcholine, a polar head group found in the phospholipids that form the bi-layers that make up cell membranes. PC is zwitterionic; that is, it contains both positive and negative charges, but is overall electrochemically neutral within a wide pH range. The high polarity of the PC group results in a tightly bound water layer, which means that, for materials containing PC, there is effectively an energy barrier reducing the likelihood of irreversible protein adsorption.
Reduced protein and cellular deposition
Invariably, when a foreign material is placed in the body it immediately begins to reject the object through the deposition of lipids and proteins, which denature, adopting active conformations that encourage the adhesion of cells from the surrounding environment. These cells may then promote further fouling of the surface.
In the case of blood, plasma proteins become irreversibly bound to the surface, followed by platelet adhesion and activation, leading to the release of additional activating factors and eventually the formation of blood clots or thrombi. By inhibiting the initial adsorption of proteins, PC materials provide an inert, non-thrombogenic surface.
When used as a coating on a medical device the improved haemocompatibility can significantly suppress the usual response of the body to a foreign material. An appreciation of this property may be seen in clinical research work reporting a favourable effect on platelets and lower blood losses during cardiopulmonary bypass surgery1. In addition, when used as a coating on coronary guide-wires, it was found that PC aids in the prevention of thrombus formation on the guide wire tip during short term clinical use2.
Suppression of protein fouling can also lead to significant reductions in the adhesion of bacterial cells and to reduced giant cell adhesion and activation, thereby potentially lowering the risk of biofilm formation and inflammation.
PC Technology materials have also been shown to reduce the adhesion of therapeutic protein to both glass and plastic surfaces and may, therefore, offer value in packaging and delivery applications.
We invite questions and discussions about our technology.
References
1. De Somer, F et al., Perfusion, 2002 Jan; 17(1):39-44.
2. Gobeil, F. et al., Canadian J. Cardiology, 2002; 18(3): 263-269