Vsoft Infoware offers e-clinical products for adaptive trials in the pharmaceutical industry. Its two flagship products are Clinfoware® and VDataWare™.
E-clinical products for adaptive trials
Vsoft Infoware is a leading provider of e-clinical products and services for adaptive trials. Our fully integrated transactional system Clinfoware and reporting system VDataWare are positioned to replace complex silos-based systems into interactive and simplified integrated system to achieve the maximum success level for pharmaceuticals, biotech and CRO industries.
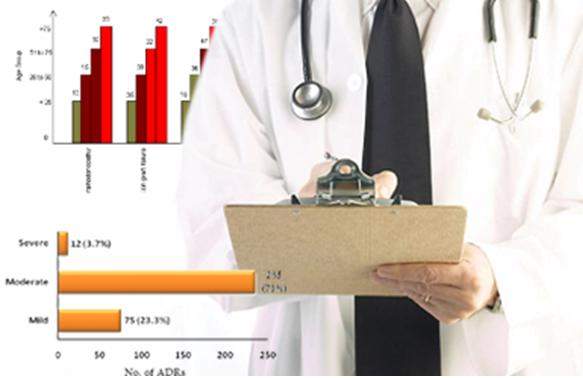
Safety surveillance, pharmacovigilance signal detection, business intelligence, and medical writing
Services provided study building and start-up, data cleaning, managing and locking, statistical analysis (including PK/PD/PG analysis and adaptive trial design) and reporting as per ICH guidelines, safety surveillance and pharmacovigilance signal detection, business intelligence, and medical writing.
These services are provided by academically qualified, certified (CCDM and SAS) and experienced professionals using our VDataWare and other tools.
Expertise in computer science and clinical operations
Vsoft has an experienced team with knowledge, expertise and qualifications in IT, computer sciences, clinical operations, including trial and data management, medical affairs, medical writing, biostatistics and SAS programming with certifications in CCDM, SAS [basic and advanced], OCPJP etc. Udison Infoware in Bangalore is an ISO 9001:2008 certified development centre that is fully owned subsidiary of Vsoft Infoware US.
Vsoft’s vision is to provide end-to-end solutions to its clients to run smooth clinical trials followed by reduced budget and duration, enhanced effectiveness of trial management, and increased clinical trial success rate.
Vsoft provides 24/7 help desk support to the global users of Clinfoware®. We support our clients with all major languages worldwide, based on their requirements. As Clinfoware® is a product to meet the demands of global trials, providing world-class support at all times has been the highest priority for Vsoft.
Electronic data capture, clinical trial management and labelling
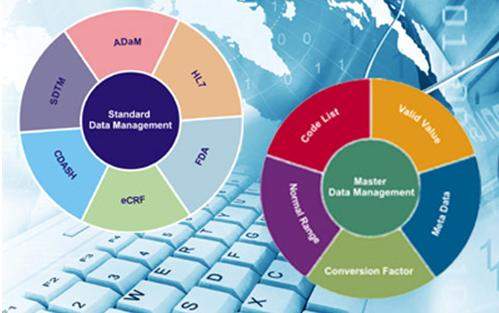
Clinfoware is fully integrated with modules such as electronic data capture, clinical trial management system, randomisation, IVRS / IWRS, clinical labelling, eDiary / ePRO, adverse event reporting system, pharmacovigilance, experimental design, imaging, data SQC, standard data management, master data management, clinical data reporting and other modules.
Clinfoware is 21 CFR Part 11 and HIPAA Compliant eClinical system, having the provision for CDASH Implementation and EHR integration. VDataWare is fully integrated with modules such as clinical study report (CSR), clinical trial report (CTR), and other modules. Vsoft also provides some of the supporting modules to its core systems that include medical writing, standard data management (SDM) and master data management (MDM).
Vsoft has successfully delivered some of its core modules of Clinfoware to many global pharma companies, with a list available on request. Vsoft has also provided various kinds of services to global pharma companies, with a list also available upon request.
Vsoft Infoware was established in 1996 headquartered in Princeton, New Jersey. It also has offices elsewhere in the US and India.